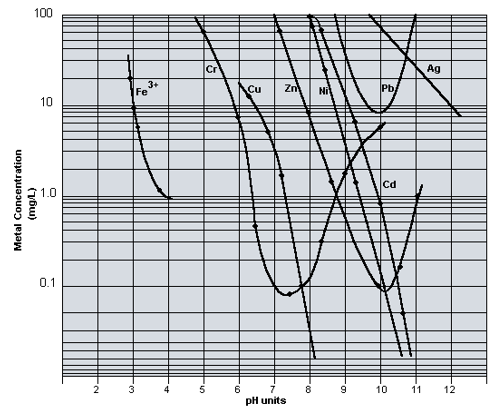
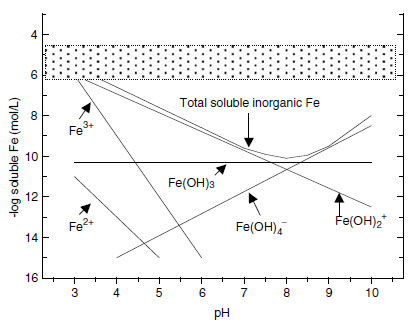
The confusion in terms is mixing chemistry involved.
Example is Sodium Chloride.
When not in water or dry, is a crystal [does not pass an electrical current]
Put NaCl in water, it dissolves to form an Electrolyte. [Or will pass an electric current]
Na [-1] + Cl [+1] + H2O
So we need the Electrolyte to be part of the Galvanic Corrosion Electrochemical Circuit.
Jim...
PS: Distilled water has no electrolytes and will NOT pass an electric current.
Example is Sodium Chloride.
When not in water or dry, is a crystal [does not pass an electrical current]
Put NaCl in water, it dissolves to form an Electrolyte. [Or will pass an electric current]
Na [-1] + Cl [+1] + H2O
So we need the Electrolyte to be part of the Galvanic Corrosion Electrochemical Circuit.
Jim...
PS: Distilled water has no electrolytes and will NOT pass an electric current.