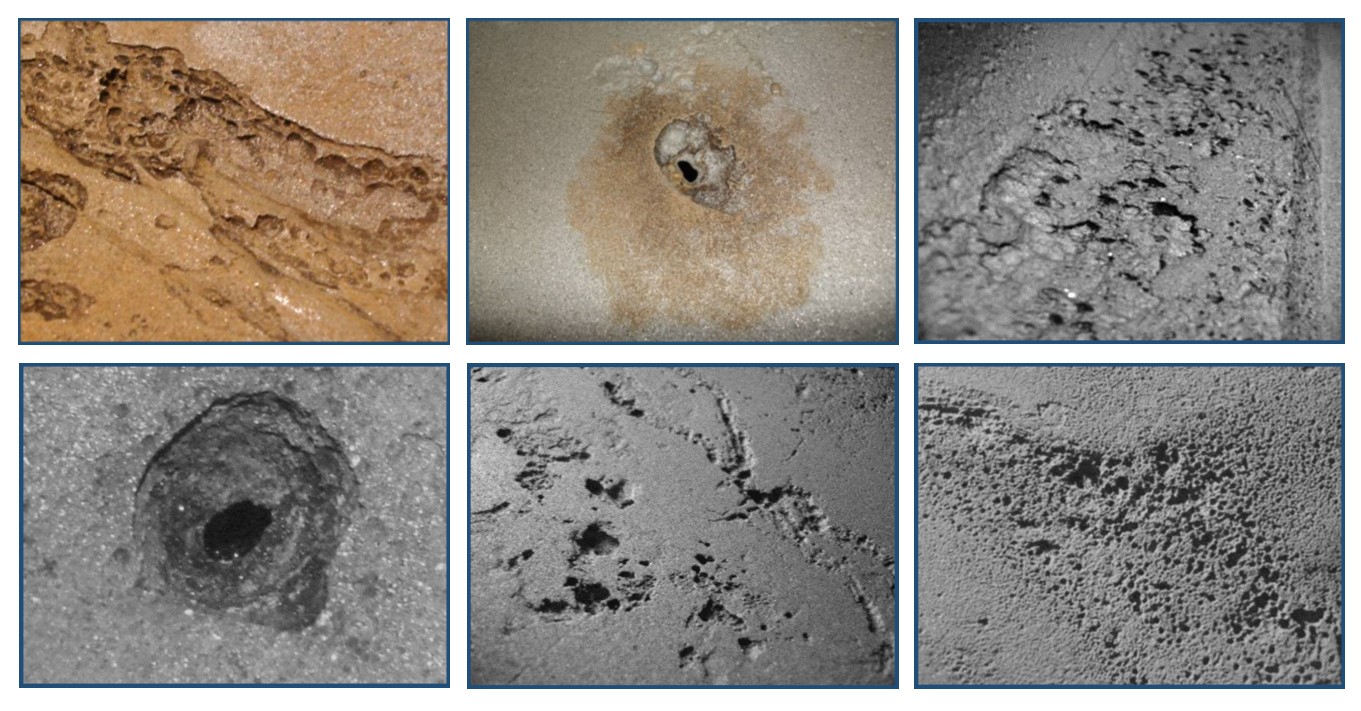
Welcome to Section 2 of our SBO Book club study of Everett Collier's book, 'The Boatowner's Guide to Corrosion".
We are reading and posting, in sections, as we work our way through the subject of corrosion in the marine environment. Each section will cover one or more chapters and be posted in its own thread. As the new threads are started, I will tag any interested participants and create a linked table of contents to make it easier to follow along and participate.
A rough outline of the sections to come are as follows. They may be edited and updated as the current of our discussion requires.
Sections
Tagged participants:
@Will Gilmore
@DArcy
@mermike
@jssailem
@dlochner
@dLj
@JamesG161
@ontherocks83
@rgranger
@Mark Maulden
@Davidasailor26
@LeslieTroyer
I'll be happy to edit/update this list at any time.
Some basic rules for maintaining useful and topic focused discussion:
SBO is a public forum, and as such, anyone interested, is welcome to participate, ask questions and express their opinions. There are limitations to the form these expressions can take and the culture of participation. All the basic forum rules of decorum and good manners apply. As a participant in this series of discussions, we would ask that participants remain on topic and refrain from derogatory language or remarks. The express purpose of this series of threads will be to understand corrosion in the marine environment using Collier's book as a guide. We therefore, expect participants to make an honest effort to read and stay up on the material under discussion. We are a group of congenial sailors with a sense of humor that often can run astray. A playful comment on occasion is expected, but anyone of us will feel free to firmly redirect anyone back to the subject at hand if it looks like it is in danger of derailing the discussion. A moderator will be asked to intervene, edit out any inappropriate comments, and possible ban an offending participant, should the group find their continued presence a serious distraction. Posting in this thread will be considered agreement to these terms. Thank you so much for your understanding and cooperation. I look forward to being part of the amazing community that is developing around this subject. I know we will all have a great time.
-Will (Dragonfly)
We are reading and posting, in sections, as we work our way through the subject of corrosion in the marine environment. Each section will cover one or more chapters and be posted in its own thread. As the new threads are started, I will tag any interested participants and create a linked table of contents to make it easier to follow along and participate.
A rough outline of the sections to come are as follows. They may be edited and updated as the current of our discussion requires.
Sections
- Sailboat Owner's Guide to Corrosion - Fundamentals (Collier 1-4)
- Sailboat Owner's Guide to Corrosion - Self-Corrosion (Collier 5)
- Sailboat Owner's Guide to Corrosion - Galvanic and Stray Current Corrosion (Collier 6 & 7)
- Sailboat Owner's Guide to Corrosion - Metals Aboard (Collier 8 - 12)
- Sailboat Owner's Guide to Corrosion - Protection (Collier 13 - 15)
- Sailboat Owner's Guide to Corrosion - Hull and Motor (Collier 16 - 17)
- Sailboat Owner's Guide to Corrosion - Electronics and Plumbing (Collier 18 - 19)
- Sailboat Owner's Guide to Corrosion - Topsides (Collier 20 - 21)
- Sailboat Owner's Guide to Corrosion - Resource
Tagged participants:
@Will Gilmore
@DArcy
@mermike
@jssailem
@dlochner
@dLj
@JamesG161
@ontherocks83
@rgranger
@Mark Maulden
@Davidasailor26
@LeslieTroyer
I'll be happy to edit/update this list at any time.
Some basic rules for maintaining useful and topic focused discussion:
SBO is a public forum, and as such, anyone interested, is welcome to participate, ask questions and express their opinions. There are limitations to the form these expressions can take and the culture of participation. All the basic forum rules of decorum and good manners apply. As a participant in this series of discussions, we would ask that participants remain on topic and refrain from derogatory language or remarks. The express purpose of this series of threads will be to understand corrosion in the marine environment using Collier's book as a guide. We therefore, expect participants to make an honest effort to read and stay up on the material under discussion. We are a group of congenial sailors with a sense of humor that often can run astray. A playful comment on occasion is expected, but anyone of us will feel free to firmly redirect anyone back to the subject at hand if it looks like it is in danger of derailing the discussion. A moderator will be asked to intervene, edit out any inappropriate comments, and possible ban an offending participant, should the group find their continued presence a serious distraction. Posting in this thread will be considered agreement to these terms. Thank you so much for your understanding and cooperation. I look forward to being part of the amazing community that is developing around this subject. I know we will all have a great time.
-Will (Dragonfly)