{This thread has lead to a larger treatment of this issue on a new thread based upon Everette Collier's book, 'The Boat Owner's Guide to Corrosion'. If you are interested in joining us, here is the link to the book club page(s):
Section 1: Sailboat Owner's Guide to Corrosion - Basic Theories (Collier 1-4)}
-
This subject often comes up as a result of one question or another, someone's prop is pitting, they need to replace their zinc or, in another current thread, are wondering about the wisdom of using a zinc coated brass fitting below the waterline.
I know some general theories of electricity, but have very little practical experience. I am very interested in learning more and I think there are others who feel the same. So, I'm starting this thread to help in understanding the nature and practices around galvanic action and electrolysis aboard.
My understanding of the issue, so that someone who actually knows something can have a place to start and help correct some misunderstandings. This is an issue with direct current, not so much with alternating current, although, it seems like half the time, alternating current could still play a roll.
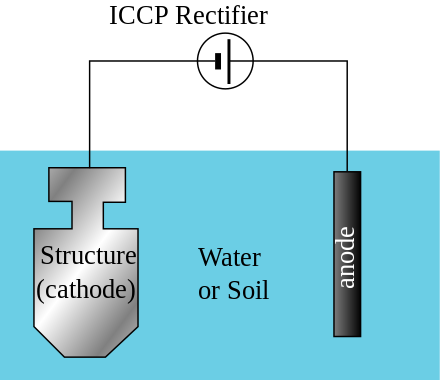
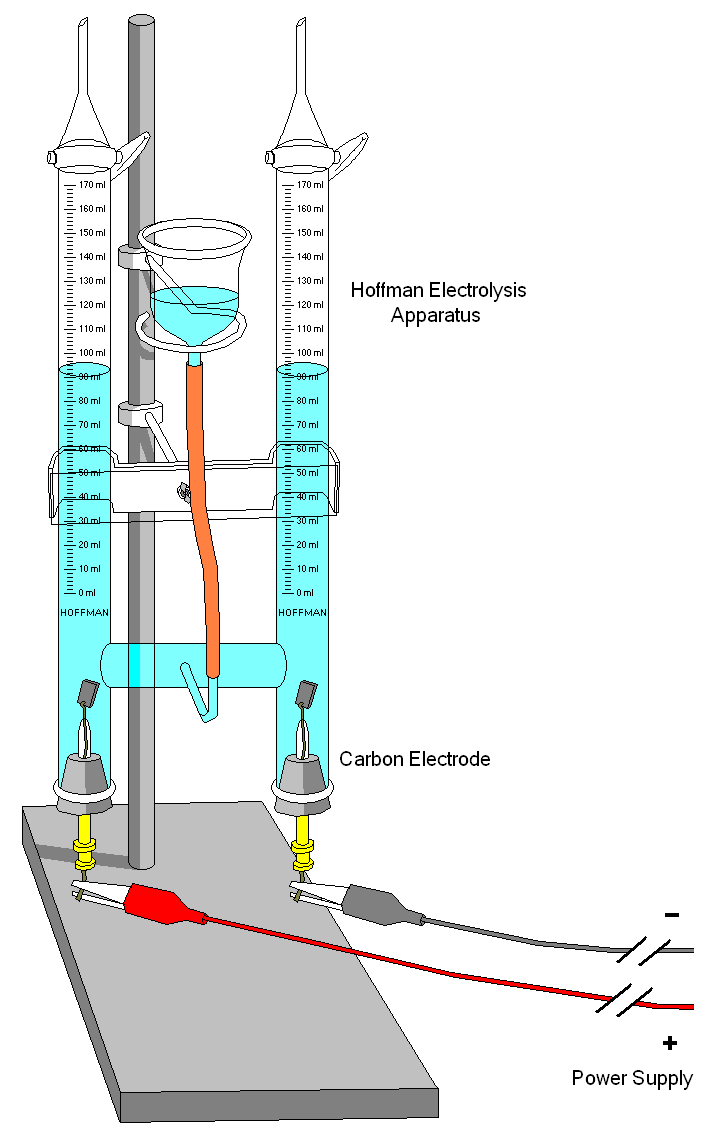
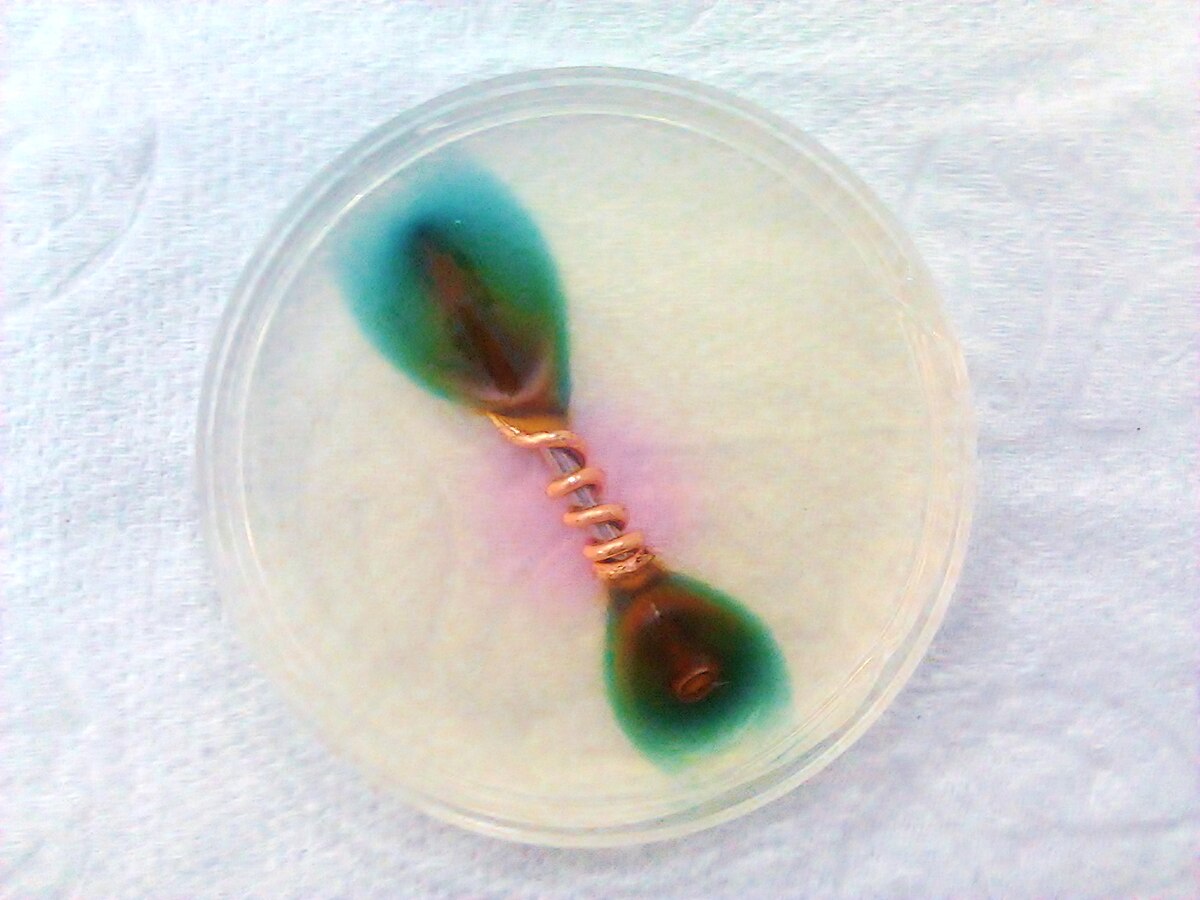
How I understand electrolysis and electricity to work, is that the less nobel metal has a looser electrical bond to its electrons and thus, in the presence of a high electrical gradient of pressure, will give up those electrons to the more nobel metal. This lose of electrons means a breaking down of the material at an atomic level. In order to do this, there needs to be a conduit or conductive pathway. This is provided by the electrolytic (i.e. saltwater). There also needs to be the electrical pressure. This is a natural gradient between the two metals, but it is made much greater, allowing the movement of electrons to overcome the material resistance between the two metals, when there is a low resistance connection to ground. This makes the connection for a completed circuit. The material that gives up electrons in the anode and the material that greedily sucks them in is the cathode.
I have comme to these ideas from reading about solutions to galvanic action by isolating boats from marina ground and their own electrical systems. It makes sense, however, that a combination of metals that naturally makes a battery, should be capable of creating an independent circuit where the solution they were immersed in allowed the flow of electrons. Another solution I have read about, is to cut off the pathway by coating the metals in teflon or other high resistance insulating materials. Shellac is another insulating material to prevent the loss of electrons. Besides providing sacrificial metals, like zinc, to prevent galvanic material lose, charging a metal with extra electrons should have the same effect. Setting up an electrical anode should take the place of a chemical anode without lose of the anode.
DC Electricity moves at about half the speed of light over a copper wire, but electrons themselves only move a few millimeters per second on average (drift velocity). It is the electron that flows and represents a negative charge. Positive charge is represented by protons, which are much larger and usually fixed in place. Ionized air is filled with electrons and is negatively charged, for example. When the electrical pressure becomes great enough between that ionized air and the positively charged ground, lightning happens.
Ground is a concept representative of a large positive charge. A collection of negative charge (electrons) will be attracted to positive charge. Elections also repel each other, again forcing movement towards the positive charge, but also, significantly, away from each other. This means what multiple pathways can and probably will be taken, as electrons moving along a branch conduit wil choose the path with the least electrons already on the branch ahead.
It seems there are a number of solutions for dealing with galvanic action. More often than not, the only two solutions discussed by sailors are, keeping metals homogeneous and/or adding a sacrificial anode into the mix.
How about it? What's going on with your electrolysis?
-Will (Dragonfly)
Section 1: Sailboat Owner's Guide to Corrosion - Basic Theories (Collier 1-4)}
-
This subject often comes up as a result of one question or another, someone's prop is pitting, they need to replace their zinc or, in another current thread, are wondering about the wisdom of using a zinc coated brass fitting below the waterline.
I know some general theories of electricity, but have very little practical experience. I am very interested in learning more and I think there are others who feel the same. So, I'm starting this thread to help in understanding the nature and practices around galvanic action and electrolysis aboard.
My understanding of the issue, so that someone who actually knows something can have a place to start and help correct some misunderstandings. This is an issue with direct current, not so much with alternating current, although, it seems like half the time, alternating current could still play a roll.
How I understand electrolysis and electricity to work, is that the less nobel metal has a looser electrical bond to its electrons and thus, in the presence of a high electrical gradient of pressure, will give up those electrons to the more nobel metal. This lose of electrons means a breaking down of the material at an atomic level. In order to do this, there needs to be a conduit or conductive pathway. This is provided by the electrolytic (i.e. saltwater). There also needs to be the electrical pressure. This is a natural gradient between the two metals, but it is made much greater, allowing the movement of electrons to overcome the material resistance between the two metals, when there is a low resistance connection to ground. This makes the connection for a completed circuit. The material that gives up electrons in the anode and the material that greedily sucks them in is the cathode.
I have comme to these ideas from reading about solutions to galvanic action by isolating boats from marina ground and their own electrical systems. It makes sense, however, that a combination of metals that naturally makes a battery, should be capable of creating an independent circuit where the solution they were immersed in allowed the flow of electrons. Another solution I have read about, is to cut off the pathway by coating the metals in teflon or other high resistance insulating materials. Shellac is another insulating material to prevent the loss of electrons. Besides providing sacrificial metals, like zinc, to prevent galvanic material lose, charging a metal with extra electrons should have the same effect. Setting up an electrical anode should take the place of a chemical anode without lose of the anode.
DC Electricity moves at about half the speed of light over a copper wire, but electrons themselves only move a few millimeters per second on average (drift velocity). It is the electron that flows and represents a negative charge. Positive charge is represented by protons, which are much larger and usually fixed in place. Ionized air is filled with electrons and is negatively charged, for example. When the electrical pressure becomes great enough between that ionized air and the positively charged ground, lightning happens.
Ground is a concept representative of a large positive charge. A collection of negative charge (electrons) will be attracted to positive charge. Elections also repel each other, again forcing movement towards the positive charge, but also, significantly, away from each other. This means what multiple pathways can and probably will be taken, as electrons moving along a branch conduit wil choose the path with the least electrons already on the branch ahead.
It seems there are a number of solutions for dealing with galvanic action. More often than not, the only two solutions discussed by sailors are, keeping metals homogeneous and/or adding a sacrificial anode into the mix.
How about it? What's going on with your electrolysis?
-Will (Dragonfly)