Unless the AC current is semi-rectified by sea water or your Negative Ground on Battery side.AC current normally has little impact as stray current corrosion
Or even the neighboring boat, metal hull boats or non 60Hz AC systems like 50Hz.
Jim...
Unless the AC current is semi-rectified by sea water or your Negative Ground on Battery side.AC current normally has little impact as stray current corrosion
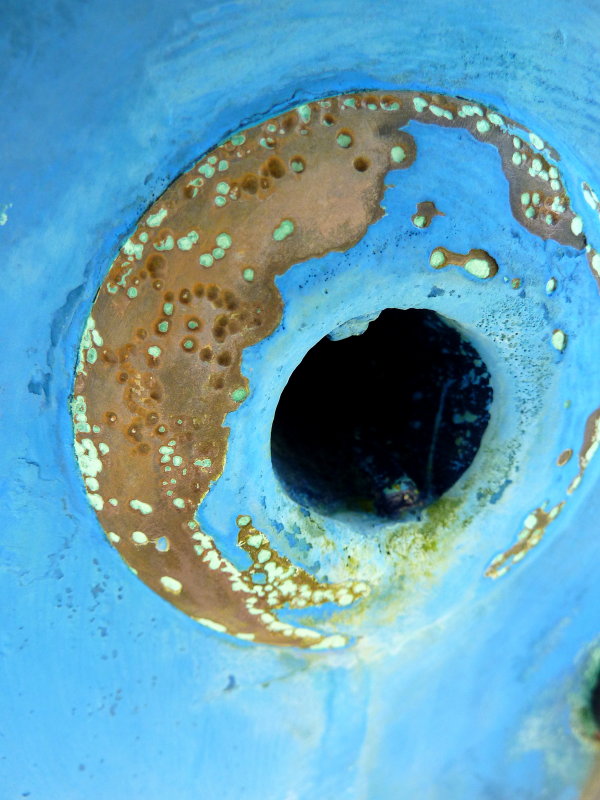
I peeked in at that thread. I would GUESS, the pin hole was caused from the inside, but too many chimed in with more info.for reference, the tank spent time sitting in a small amount of water right in that corner
The photos you posted in your thread on your tank showed corrosion initiation from the outside surface of the tank rather than from the inside of the tank. However, your images from the inside of the tank, while not very clear due to the geometric limitations, did show a fair amount of what appeared to be deposits on the inner surfaces. That can be a bit sketchy to evaluate without more detailed examination but the conservative approach is to simply replace the tank.Hey! That's my tank!
for reference, the tank spent time sitting in a small amount of water right in that corner. the water was in a fiberglass basin with nothing else, no grounds or anything.
I'm going with isolation and an additional barrier coat on the bottom of the tank combined with rubber standoffs and a drain drilled into the cradle so that if any water does get in there, it gets out immediately. The corrosion was outside -> in for sure.You will need to address the conditions that caused your current problem - be that make a cradle to pick your tank up so it's not sitting in water, or somehow getting rid of that problem so water can't sit on the tank. A cradle sounds like a good option to me, but it's your boat, your choice...
dj
Sure - at 30 amps per square meter and above! That's big current! The article you are referencing is dealing with railways and high power transmission lines, especially inthe effect on the corrosion of pipelines that may be crossing or running parallel to those high power transmission sources. And then at those power levels, the corrosion rate is doubled from the corrosion rate if that were not present. So that rate is not a lot, and the current required is quite large. So, don't park your boat on an electrified railroad track...Here's an excerpt from an abstract to a research study on AC stray current corrosion:
Study on the Influence of AC Stray Current on X80 Steel under Stripped Coating by Electrochemical Method
"Goidanich et al. [15] obtained an experimental result that the AC corrosion rate is twice the self-corrosion rate of the undisturbed current sample at a current density of 10A/m2; when the AC current density exceeds 30A/m2, the corrosion rate increases exponentially."
I hope I'm not taking this out of context and completely misrepresenting the conclusion, but it looks like the AC stay current has a definite negative effect on corrosion rates.
-Will (Dragonfly)
Ok but we are talking perhaps even a trickle of Amps.It's simply not the major player in what we are talking about - boats. Of course one must install AC power correctly...
MilliAmps / Zinc anode surface areas.
Is BIG...
So...
Avoid All Stray Currents to reduce Corrosion rates...
Jim...
Yes, that is the point. Clearly defined definitions and terms are important to that end. I hope to have the time to compile a list of important key terms to define for each section. I will edit them into the introduction post at the beginning of the thread and list, perhaps, in their entirety in the Resource thread. I look to you guys for any help in all of this.It is my understanding that these threads are aimed at bringing greater clarity to to the subject of corrosion as it applied to our boats, I feel strongly that mixing up terminology, something Collier does way too much in his book, should be avoided.
+1..! Here's what DC stray current can do in less than 24 hours.....I'm not saying that AC current doesn't have any effect on stray current corrosion. It's simply not the major player in what we are talking about - boats. Of course one must install AC power correctly...
dj

That is a significant point to make. Kind of a myth busters conclusion. Thanks for chiming in.We find just as much DC stray current issues on mooring sailed boat as we do on marina sailed boats.
The vast majority of DC stray current issues originate on the boat not from off the boat. Unfortunately most boat owners assume all corrosion is caused by being in a marina.That is a significant point to make. Kind of a myth busters conclusion. Thanks for chiming in.
-Will (Dragonfly)
Or a new boat that just entered the marina.Unfortunately most boat owners assume all corrosion is caused by being in a marina.
Please note the "pitting" on that picture. Plus the Green Copper residue from the Bronze alloy decomposing.thru-hull fitting that was in contact with water was destroyed.
Will, why would you create a new definition for these terms? NACE has published definitions.Galvanic Corrosion: Corrosion of an anode metal due to its electrical connection to a more noble metal, the cathode, while immersed in an electrolyte.
Stray Current: In the world of corrosion, stay current reffers to an electrical current that promotes corrosion and whose source is external to the galvanic cell created by electrically connected disparate metals in an electrolyte.
What do you guys think? Two working definitions for this section. Any tweaks or additions?
I'll put others up when I can.
Anode - section 1
Cathode - section 1
Electrolyte - secretion 1
Pit Corrosion - section 2
Crevice Corrosion - section 2
I'm taking suggestions.
-Will (Dragonfly)
Will,See, that's just the kind of help I'm looking for.
-Will (Dragonfly)
